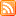Molecular composition of DIRT
11 years ago chemistry, dirt, soil composition
While walking down the hallways at work I noticed a coworker with a caffeine molecular T-Shirt and made me think of the question, “What does a simple spec of dirt look look?” I know my brain works in weird ways…but anyway what I found was pretty amazing. Above is what an average molecule’s chemical composition looks like.
For any of you chemistry nerds more specifically this is C349H401N26O173S which is visually represented above.
Humic Acid
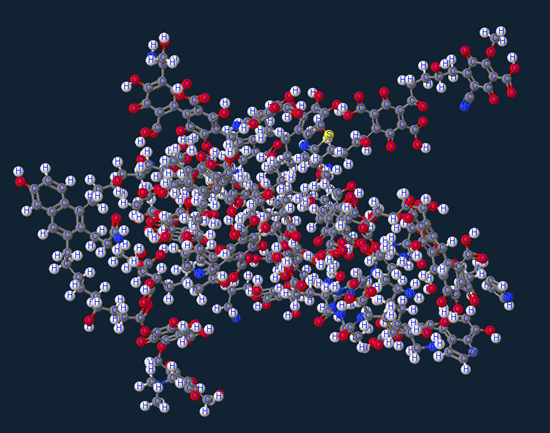
The majority of this molecule is Humic acid which comprises of the majority of organic soil matter (or dirt) which is a very weak acid group partially visualized below as groupings of Carboxylic/Phenolic/Alcoholic/Quinonic/Ketone/Methoxl.
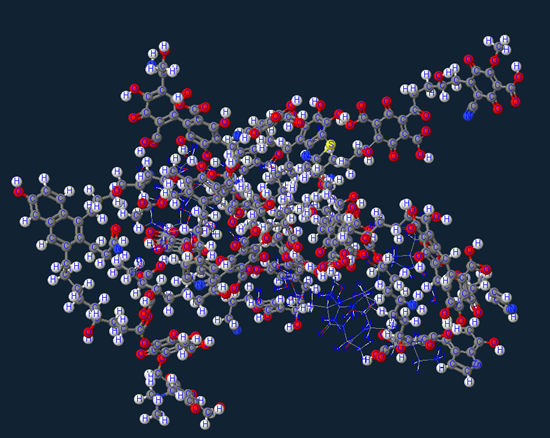
Saccharides (sugars)
Next we have the saccharides or as we more commonly refer to as sugar…
Proteinaceous material
I don’t even know where to start on this amazing hexapeptide…so I will jump to the pretty picture.
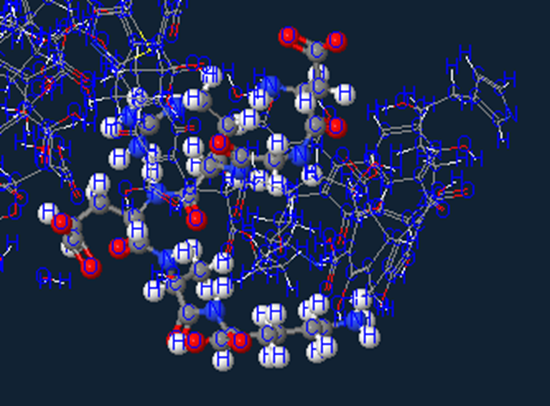
Water
Of course you also have to have some good ole’ H20
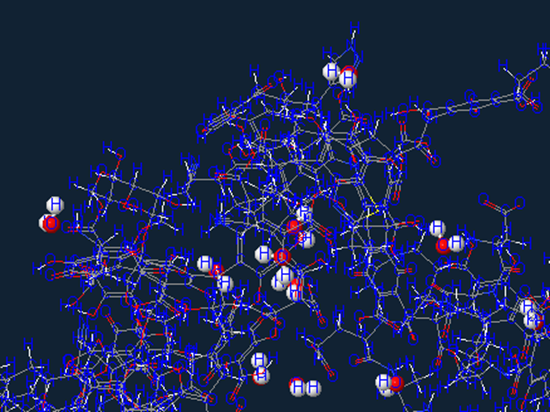
Aromatic carbon
Which is a hydrocarbon with alternating double and single bonds between carbon atoms forming rings.
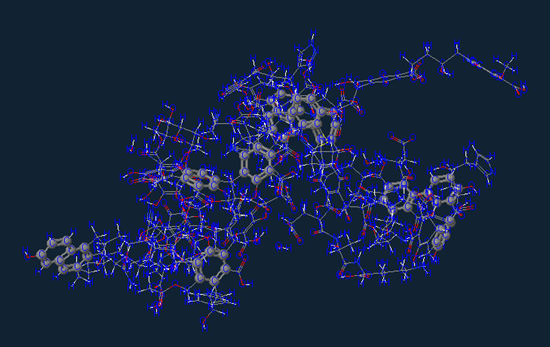
Now of course not every piece of dirt appears exactly like what is shown above, but this would be the more common of the bunch. Depending on how you compost and/or sourced your soil you will also have all kinds of neat trace minerals in your soil that will help your plants grow but also provide digestible nutrients to the foods you grow.
So next time you pick up a handful of soil you can have a even greater appreciation for the cool chemistry that made it all possible.